Production in accordance with pharmaceutical standards/GMP
The quality of our herbal medicines is at the highest level: from the natural raw material to the finished medicinal product, Harras Pharma Curarina Arzneimittel GmbH is a one-stop service. The sister company Gehrlicher Extrakte is a GMP-certified manufacturing company for medicinal herbal extracts.
Approved natural medicinal products
Our herbal medications are approved and registered by regulatory authorities at home and abroad. Depending on the country, there are special requirements regarding the verification of quality, efficacy and safety before medicinal product approval can be granted.
Active herbal ingredients for herbal medicines (DMF or ASMF)
The healing powers of herbal active ingredients have been proven in herbal therapy for generations. In recent decades, standardisation and improved quality control have brought about a high pharmaceutical standard in herbal pharmacy. Nowadays, pharmaceutical herbal extracts require a Drug Master File (DMF) or Active Substance Master File (ASMF).
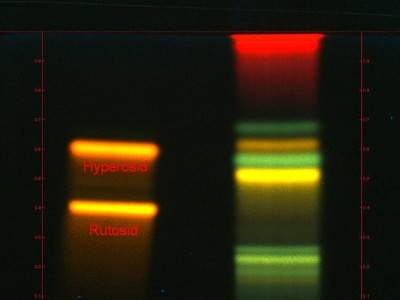
Standardised extracts
As is the case with all natural products, a range of environmental factors, soil and climate for example, influence the growth of medicinal plants, so that the active ingredient potency can vary from harvest to harvest. Nonetheless, with modern analysis methods, a consistent high quality can always be guaranteed. This standardisation of extracts guarantees the consistent potency of the active ingredient and is an important criterion for quality assurance of the Harras Pharma Curarina agents.
Controlled quality from the plant to the finished medicinal product
In order to ensure that the high quality standard of Harras Pharma Curarina preparations remain guaranteed, the plant raw materials have to meet the strictest requirements. Thanks to its long experience, Harras Pharma Curarina has proven sources for purchasing the herbal material, and strictly controls the quality of the raw material.
For certain preparations, the company grows its own medicinal plants. This means: controlled quality – from the raw material on.
Monitored, controlled and certified by regulatory authorities: GMP and GDP
Our extraction plant in Eurasburg and the medicinal products distribution in Munich are regularly inspected and monitored by the regional government of Upper Bavaria. Medicinal products production is certified according to international GMP (Good Manufacturing Practice) standards. For the trading with medicinal products, we have the wholesale permit GDP (Good Distribution Practice).
Harras Pharma GmbH is a member of the BAH
By company membership, the German Medicines Manufacturers’ Association (BAH) is the leading trade organisation for the pharmaceutical industry in Germany. About the association…